Abstract
Background: CTL019 (tisagenlecleucel) is an adoptive cellular immunotherapy that uses the autologous peripheral blood T cells that have been genetically modified ex vivo to target CD19 on the surface of B cells. To date, little is known about the effect of cellular kinetics on clinical response, dose-response, and safety of CTL019 in DLBCL patients.
Methods: Data from a pivotal phase 2 study (JULIET; NCT02445248) in r/r DLBCL patients were utilized to characterize exposure-response, dose-response, and dose-safety analyses of CTL019 in DLBCL patients. The cellular kinetic parameters were estimated from non-compartmental analysis (NCA) utilizing the time course of CTL019 transgene levels in peripheral blood, as determined using quantitative real-time polymerase-chain-reaction (qPCR) assay, following CTL019 infusion. The descriptive summary statistics for the cellular kinetic parameters (AUC, Cmax, Tmax, and Tlast) were compared between responder (CR and PR) and non-responder patients (SD, PD, and unknown response status) to investigate the relationship between exposure and response at month 3. In addition, the cellular kinetics were compared between DLBCL and pediatric ALL patients to assess the indication-specific differences in CTL019 expansion in vivo .
To assess the impact of dose on month 3 tumor response, duration of response (DOR), and safety (CRS and neurologic event, any grade and grade 3 or 4), logistic regression or Cox regression analyses were performed.
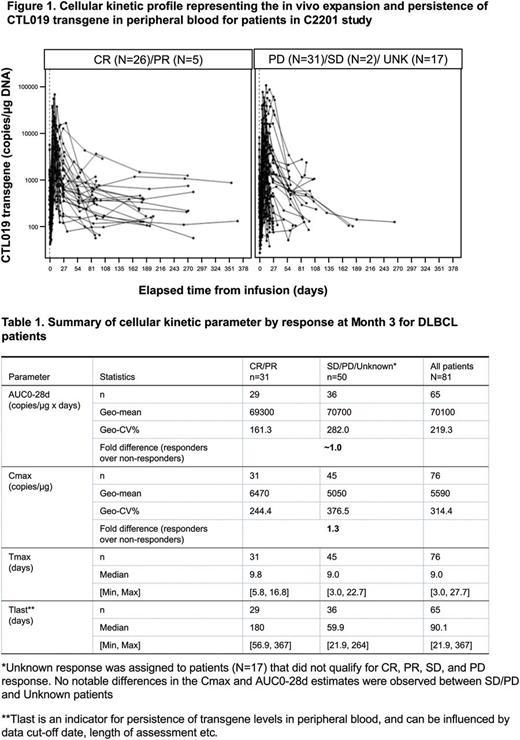
Results: Data from 99 patients (JULIET) were utilized for the analysis. The cellular kinetic parameters were summarized for a subset (N=81) of the data obtained through serial measurements in peripheral blood using qPCR assay. The geometric mean AUC0-28d and Cmax values and median Tmax in responder patients (CR and PR) were similar to those in non-responding patients, suggesting similar levels of in vivo expansion in responding and non-responding r/r DLBCL patients (Figure 1, Table 1). Most of the patients with longer follow up, as of the data cutoff date (8 March 2017), continue to demonstrate detectable transgene levels in peripheral blood. The geometric mean estimate for expansion (Cmax) in DLBCL patients were observed to be 6-fold lower than that in pediatric ALL patients, (Mueller KT, et al. EHA 2017) indicating possible indication-specific differences in expansion in peripheral blood. Higher expansion were associated with higher CRS grades. No obvious relationship between dose and exposure (AUC0-28d and Cmax) was observed. Across the wide range of dose administered, the logistic regression dose response curve showed that there is no apparent impact of dose on response at month 3 (two-fold increase in dose associated with odds ratio (OR): 1.03; 95% CI: 0.624, 1.685). Similarly, Cox regression showed no apparent impact of dose on DOR. The analysis showed that the probability of CRS increases with increase in the dose (any grade and grade 3 or 4) (two-fold increase in dose associated with OR = 2.79 for any grade CRS, 95% CI: 1.394, 5.567). The logistic regression results indicated that there is no obvious impact of dose on the neurological event.
Conclusion: The differences in expansion levels between two indications (DLBCL and pediatric ALL) in peripheral blood highlight the mechanistic differences in CTL019 expansion based on the tumor type. CTL019 demonstrates efficacy across the dose range evaluated with no obvious impact of dose on neurologic events. An increased probability for higher grade CRS with increased dose was observed; however, CRS is generally manageable with appropriate staff training. These analyses provide insights into the relationship among exposure, response, dose and safety endpoints for CAR therapy in the DLBCL indication.
Awasthi: Novartis Pharmaceuticals Corporation: Employment. Tam: Abbvie: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding; Roche: Honoraria, Research Funding. Jaeger: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jaglowski: Pharmacyclics: Consultancy, Research Funding. Foley: Novartis Pharmaceuticals Corporation: Consultancy. Wagner-Johnston: Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Kersten: MSD: Honoraria; BMS: Honoraria; Gilead Sciences: Honoraria; Mundipharma: Honoraria; Milennium/Takeda: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Kite Pharma: Honoraria; Amgen: Honoraria; Novartis Pharmaceuticals Corporation: Honoraria. Schuster: Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy; Nordic Nanovector: Consultancy; Merck: Research Funding; Gilead: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Salles: Amgen, BMS, Celgene, Gilead, Janssen, Kite, Merck & Co., Inc., Morphosys, Novartis, Roche, Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding. Maziarz: Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria; Juno Therapeutics: Honoraria; Kite Therapeutics: Honoraria; Athersys, Inc: Patents & Royalties. Anak: Novartis Pharma AG: Employment. Bubuteishvili Pacaud: Novartis Pharma AG: Employment. Gazi: Novartis Pharma AG: Employment. Waldron: Novartis Pharmaceuticals Corporation: Employment. Hamilton: Novartis Pharmaceuticals Corporation: Employment. Pruteanu: Novartis Pharmaceuticals Corporation: Employment. Tai: Novartis Pharmaceuticals Corporation: Employment. Mueller: Novartis Pharmaceuticals Corporation: Employment. Waller: Cambium Medical Technologies: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; AMGEN: Consultancy; Coulter Foundation: Research Funding; Celldex: Consultancy; Chimerix: Equity Ownership; Novartis Pharmaceuticals Corporation: Consultancy, Honoraria, Research Funding; National Institutes of Health: Research Funding; Katz Foundation: Research Funding; PRA: Consultancy; Cerus: Equity Ownership; Helocyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal